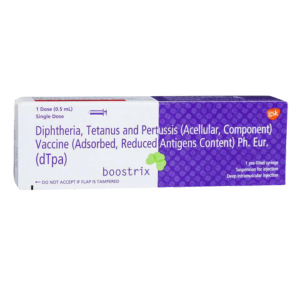
TDaP Vaccine-Boostrix
₹1,535.00
Indication: For active immunization against tetanus, diphtheria, and pertussis (whooping cough) in individuals aged 10 years and older.
Route: Administered as an intramuscular injection, typically in the upper arm.
Immunization Schedule: Given as a single booster dose, with additional booster doses as recommended by a healthcare provider for continued protection.
Out of stock
Description
Comprehensive FAQs on “TDaP Vaccine – BOOSTRIX (Tetanus, Diphtheria, and Pertussis Vaccine)
1. What is BOOSTRIX used for?
Answer: BOOSTRIX is used as a booster vaccine to protect against tetanus, diphtheria, and pertussis (whooping cough) in individuals aged 10 years and older. It is also recommended for pregnant individuals during the third trimester to help protect newborns from pertussis.
2. Who should receive the BOOSTRIX vaccine?
Answer: BOOSTRIX is recommended for adolescents, adults, and pregnant individuals who need a booster dose to maintain immunity against tetanus, diphtheria, and pertussis. It is especially beneficial for individuals with incomplete vaccination histories or for pregnant women to protect their newborns.
3. What is the immunization schedule for BOOSTRIX?
Answer: BOOSTRIX is given as a single booster dose at least 5 years after the last dose of a DTaP or Td vaccine. An additional dose may be given 9 years or more after the initial Tdap dose. It is also recommended for tetanus prophylaxis in wound management when 5 years have passed since the last tetanus-containing vaccine.
4. How is BOOSTRIX administered?
Answer: The vaccine is administered as a 0.5 mL intramuscular injection, typically into the deltoid muscle of the upper arm.
5. Can BOOSTRIX be given to pregnant women?
Answer: Yes, BOOSTRIX is recommended during the third trimester of pregnancy to help protect infants younger than 2 months from pertussis (whooping cough) by transferring maternal antibodies.
6. What are common side effects of BOOSTRIX?
Answer: Common side effects include pain, redness, or swelling at the injection site, fatigue, headache, mild fever, and muscle pain. These reactions are generally mild and temporary.
7. Who should not receive the BOOSTRIX vaccine?
Answer: Individuals who have had severe allergic reactions (e.g., anaphylaxis) to any component of the vaccine or those with a history of encephalopathy within 7 days of a previous pertussis-containing vaccine should not receive BOOSTRIX.
8. Can BOOSTRIX be given with other vaccines?
Answer: Yes, BOOSTRIX can be administered alongside other vaccines, but they should be given at separate injection sites. Consult with your healthcare provider for specific guidance.
9. Is a booster dose of BOOSTRIX required regularly?
Answer: After the initial dose, a booster dose may be recommended every 10 years to maintain immunity, particularly for individuals at high risk of exposure or for tetanus prophylaxis in wound management.
10. How long does immunity last after receiving BOOSTRIX?
Answer: Immunity from BOOSTRIX typically lasts for about 10 years. Booster doses may be recommended based on the individual’s risk factors and exposure to tetanus, diphtheria, and pertussis.
11. Can BOOSTRIX be used for wound management?
Answer: Yes, BOOSTRIX can be administered for tetanus prophylaxis in wound management if at least 5 years have passed since the last tetanus-containing vaccine was given.
12. Can adults receive BOOSTRIX even if they have received other vaccines?
Answer: Yes, BOOSTRIX can be given to adults and adolescents, even if they have received other vaccines in the past, to maintain and boost immunity against tetanus, diphtheria, and pertussis.
13. Are there any special precautions for receiving BOOSTRIX?
Answer: Inform your healthcare provider if you have any severe allergies, a history of Guillain-Barré syndrome, or any neurological disorders. If you are experiencing a moderate or severe illness, vaccination should be delayed until recovery.


 Book a Test
Book a Test
 Download Reports
Download Reports
 Health Packages
Health Packages
 Home Med Service
Home Med Service

