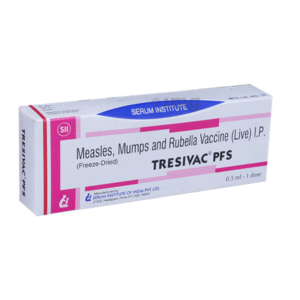
MMR Vaccine-Tresivac PFS
₹660.00
Indication: For active immunization against measles, mumps, and rubella in infants, children, adolescents, and young adults at risk.
Route: Administered as a subcutaneous injection.
Immunization Schedule: First dose at 9 months, second dose between 15-18 months, and third dose between 4-6 years of age.
Out of stock
Description
The Tresivac PFS MMR vaccine offers effective protection against measles, mumps, and rubella. This essential vaccine is suitable for both children and adults, providing immunity from these highly contagious diseases.”
Comprehensive FAQs on TRESIVAC PFS (Measles, Mumps, Rubella Vaccine)
1. What is TRESIVAC PFS used for?
Answer: TRESIVAC PFS is used for the active immunization against measles, mumps, and rubella in infants, children, adolescents, and young adults at risk of these diseases.
2. Who should receive the TRESIVAC PFS vaccine?
Answer: It is recommended for infants starting at 9 months of age, with additional doses for children, adolescents, and young adults at risk of infection.
3. What is the immunization schedule for TRESIVAC PFS?
Answer: The schedule includes three doses: the first dose at 9 months, the second dose between 15-18 months, and a third dose between 4-6 years of age.
4. How is TRESIVAC PFS administered?
Answer: The vaccine is given as a subcutaneous injection, typically in the upper arm or thigh.
5. Can adults receive the TRESIVAC PFS vaccine?
Answer: Yes, adults who have not been vaccinated or are at high risk for measles, mumps, and rubella infection can receive the TRESIVAC PFS vaccine, especially those traveling to high-risk areas or working in healthcare and educational settings.
6. How many doses are required for adults?
Answer: Adults typically need two doses of the MMR vaccine, administered at least 28 days apart, to ensure full immunity.
7. Is a booster dose required?
Answer: The standard immunization schedule generally provides lifelong immunity for most individuals. However, certain people, such as those with compromised immunity or at high risk, may need booster doses. Consult your healthcare provider for specific recommendations.
8. How can I check if the vaccine is working?
Answer: Immunity can be verified through a blood test known as a titer test, which measures the presence and levels of antibodies against measles, mumps, and rubella in the blood.
9. What are the common side effects of TRESIVAC PFS?
Answer: Common side effects include mild fever, rash, or swelling at the injection site. These effects are usually short-lived and resolve on their own.
10. Who should not receive the TRESIVAC PFS vaccine?
Answer: The vaccine should not be given to individuals with severe allergic reactions to any component of the vaccine or those with compromised immune systems without medical supervision.
11. Can I take the TRESIVAC PFS vaccine during pregnancy?
Answer: No, pregnant women should not receive the TRESIVAC PFS vaccine. It is recommended to avoid becoming pregnant for at least one month following vaccination. Consult with a healthcare provider for further advice if you are planning to become pregnant.
12. Can TRESIVAC PFS be given with other vaccines?
Answer: Yes, it can be administered alongside other vaccines, but different injection sites should be used. Always consult with a healthcare provider for specific recommendations.
13. What precautions should be taken before receiving the vaccine?
Answer: Inform your healthcare provider if you have any allergies, immune system disorders, or if you are taking medications that affect the immune system. Individuals with acute febrile illnesses should postpone vaccination.


 Book a Test
Book a Test
 Download Reports
Download Reports
 Health Packages
Health Packages
 Home Med Service
Home Med Service

