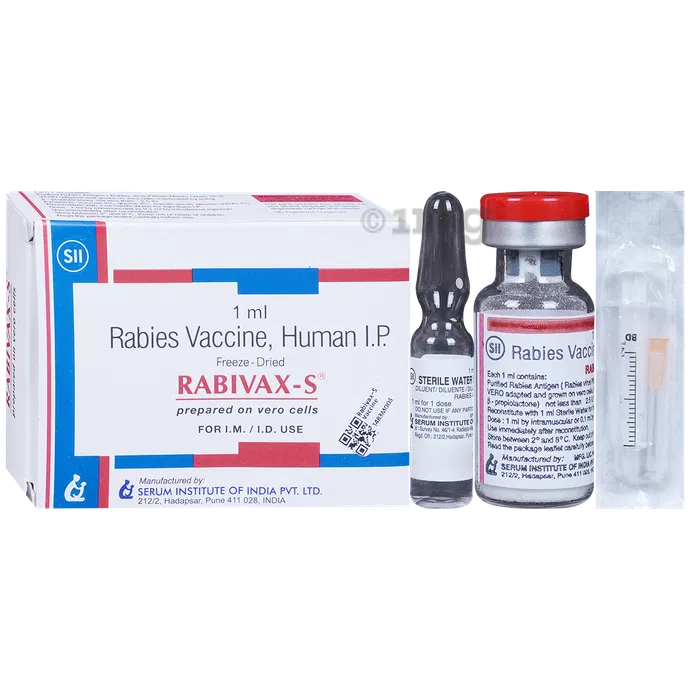
Rabies Vaccine-Rabivax S
₹380.00
Indication:
RABIVAX-S is indicated for the prevention of rabies in children and adults. It can be used for pre-exposure and post-exposure prophylaxis.
Route of Administration:
- Intramuscular (IM)
- Intradermal (ID)
Immunization Schedule:
Pre-exposure Prophylaxis (High-risk populations):
- Intramuscular: 1 ml on Days 0, 7, and 21/28.
- Intradermal: 0.1 ml on Days 0, 7, and 21/28.
Post-exposure Prophylaxis (Unimmunized individuals):
- Intramuscular: 1 ml on Days 0, 3, 7, 14, and 28.
- Intradermal: 0.1 ml on Days 0, 3, 7, and 28.
Post-exposure (Previously Immunized):
- Single Dose on Days 0 and 3 (IM: 1 ml or ID: 0.1 ml).
Out of stock
Description
FAQs on RABIVAX-S
1. What is RABIVAX-S and what is it used for?
RABIVAX-S is a rabies vaccine used for the prevention of rabies in both children and adults. It can be used as a primary immunization (pre-exposure prophylaxis) for high-risk individuals or as post-exposure prophylaxis (PEP) after a potential rabies exposure.
2. Who should receive Rabivax S Rabies Vaccine?
- Pre-exposure Prophylaxis: Individuals at high risk of rabies exposure, such as veterinarians, laboratory staff, animal handlers, travelers to rabies-endemic areas, and people working with animals.
- Post-exposure Prophylaxis: Anyone who has potentially been exposed to the rabies virus through bites, scratches, or contact with the saliva of a rabid or suspected rabid animal.
3. How is RABIVAX-S administered?
The vaccine can be administered via the intramuscular (IM) or intradermal (ID) route.
- For IM administration, the deltoid muscle is typically used.
- For ID administration, the vaccine is injected into the skin.
4. What is the recommended immunization schedule for RABIVAX-S?
- Pre-exposure Prophylaxis:
- Intramuscular: 1 ml dose given on Days 0, 7, and 21 or 28.
- Intradermal: 0.1 ml dose given on Days 0, 7, and 21 or 28.
- Post-exposure Prophylaxis (Unimmunized Individuals):
- Intramuscular: 1 ml dose given on Days 0, 3, 7, 14, and 28.
- Intradermal: 0.1 ml dose given on Days 0, 3, 7, and 28.
- Post-exposure for Previously Immunized Individuals:
- One dose on Day 0 and Day 3 (IM: 1 ml or ID: 0.1 ml).
5. Are there any side effects associated with RABIVAX-S?
Common side effects include mild pain, redness, or swelling at the injection site. Less commonly, people may experience fever, headache, dizziness, or general malaise. Serious allergic reactions are rare but require immediate medical attention
.
6. Can pregnant or breastfeeding women receive RABIVAX-S?
Yes, RABIVAX-S can be administered to pregnant or breastfeeding women if they are exposed to rabies or are at risk of exposure. The benefits of protecting against rabies far outweigh any potential risks from the vaccine.
7. What should I do if I miss a dose of RABIVAX-S in the PEP schedule?
It is important to follow the prescribed schedule closely. If a dose is missed, you should contact a healthcare professional immediately for guidance on completing the vaccination series.
8. Do I need to restart the series if a dose is delayed?
If there is a delay in the vaccination schedule, you generally do not need to restart the series. Instead, complete the missed doses as soon as possible. Always follow the guidance of a healthcare professional.
9. How effective is RABIVAX-S in preventing rabies?
When administered according to the recommended schedule, RABIVAX-S is highly effective in preventing rabies. It is critical to complete the full course for optimal protection.
10. Can RABIVAX-S be used interchangeably with other rabies vaccines?
In general, rabies vaccines are considered interchangeable; however, it is preferable to complete the vaccine course with the same brand, if possible. Consult a healthcare professional for specific guidance.
11. Can RABIVAX-S be given alongside other vaccines?
Yes, RABIVAX-S can be administered concurrently with other vaccines, but it should be given at a separate injection site. Consult a healthcare provider if you have concerns about interactions with other vaccines.
12. Do I need a booster dose of RABIVAX-S?
For individuals at ongoing risk of rabies exposure, periodic booster doses may be recommended after a serological test to measure rabies-neutralizing antibodies. Frequency depends on risk level and professional advice.
This list aims to cover common queries while providing essential guidance based on current medical recommendations for rabies prevention using RABIVAX-S. Always seek medical advice for specific individual needs.


 Book a Test
Book a Test
 Download Reports
Download Reports
 Health Packages
Health Packages
 Home Med Service
Home Med Service

